Hepatitis A Vaccination and the Universal Immunisation Programme
Context
Public health experts are recommending the inclusion of the Hepatitis A vaccine in India’s Universal Immunisation Programme (UIP) due to increasing outbreaks and diminishing natural immunity among adolescents and young adults.
About Hepatitis A
Definition
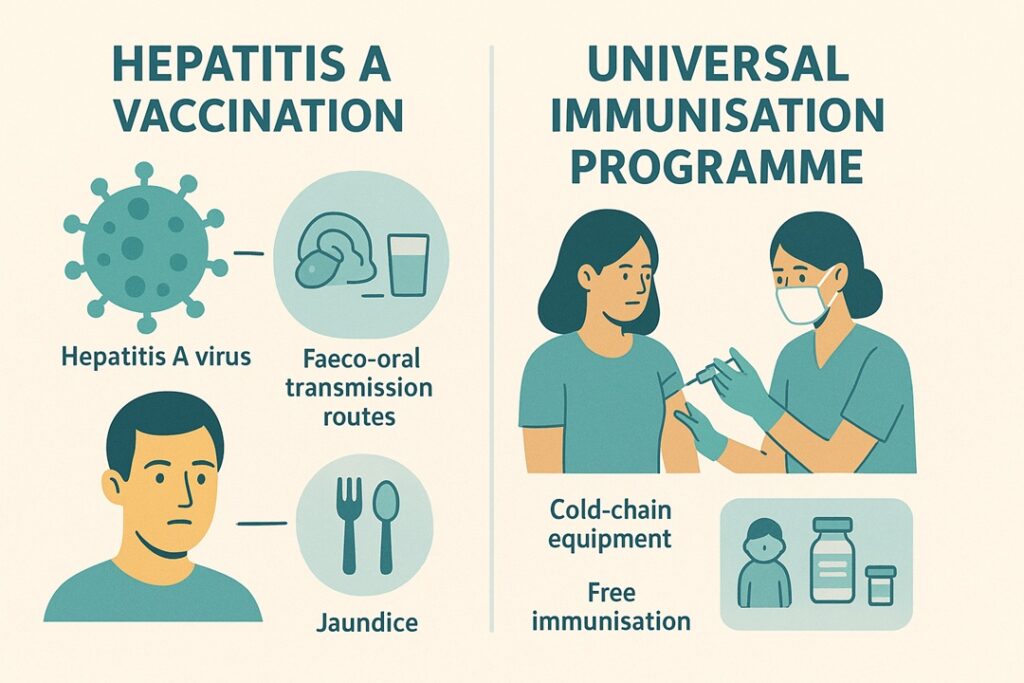
Hepatitis A is an acute viral liver infection caused by the Hepatitis A Virus (HAV). It does not lead to chronic liver disease but can result in severe hepatitis or acute liver failure, particularly in adults.
Geographical Distribution
Hepatitis A occurs globally with cyclic outbreaks and both sporadic and epidemic patterns.
It is highly prevalent in low- and middle-income regions with suboptimal sanitation, where most children acquire the infection early in life.
High-income countries have low prevalence, but outbreaks occur among specific high-risk groups such as men who have sex with men, people who inject drugs, travellers and homeless populations.
Mode of Transmission
Transmission is through the faeco-oral route.
Infection occurs via ingestion of contaminated food or water.
Direct person-to-person transmission occurs due to poor hygiene, unclean hands or oral-anal contact.
Household spread is common, especially when infected individuals handle food.
Waterborne outbreaks are linked to sewage contamination.
Symptoms
Fever, malaise, loss of appetite, nausea, diarrhoea, abdominal discomfort, dark urine and jaundice.
Treatment
No specific antiviral treatment is available.
Hospitalisation is required only for severe manifestations or acute liver failure.
Most individuals recover completely and develop lifelong immunity.
About Universal Immunisation Programme (UIP)
Definition
The Universal Immunisation Programme is India’s largest public-health vaccination initiative, offering free immunisation to children and pregnant women against major vaccine-preventable diseases. It is one of the world’s largest immunisation programmes.
Launch Timeline
1978 – Introduced as the Expanded Programme on Immunization
1985 – Expanded and renamed as the Universal Immunization Programme
1989–90 – Achieved nationwide coverage
Key Components
Strategy and Policy Framework
Guided by the National Health Policy, National Vaccine Policy and recommendations of the National Technical Advisory Group on Immunisation (NTAGI).
Cold Chain, Vaccines and Logistics
Approximately 30,000 cold chain points operate across the country.
Infrastructure includes Ice-Lined Refrigerators, Deep Freezers, Walk-In Coolers, Walk-In Freezers and dedicated vaccine vans.
The electronic Vaccine Intelligence Network ensures real-time monitoring of stock and temperature.
Injection Safety and Waste Management
Use of auto-disable syringes, hub-cutters and disposal as per Central Pollution Control Board guidelines on biomedical waste.
AEFI Surveillance
A structured multi-level Adverse Events Following Immunisation reporting system has existed since 1988.
The SAFEVAC portal enables real-time reporting and monitoring.
Training and Capacity Building
Standardised training is provided to medical officers, health workers and cold-chain technicians through institutions such as the National Cold Chain and Vaccine Management Resource Centre (Delhi) and the National Cold Chain Training Centre (Pune).
Conclusion
Incorporating the Hepatitis A vaccine under UIP can help address increasing susceptibility among adolescents and adults, reduce outbreak risks and strengthen India’s immunisation coverage.
Source : The Hindu